People who used Zantac (ranitidine) are well aware by now that in April of 2020, the U.S. Food and Drug Administration (FDA) issued a market withdrawal for the popular heartburn medication after finding high levels of a known carcinogen in testing. Prior to and after the FDA market withdrawal, Zantac manufacturers issued voluntary recalls, citing safety concerns. 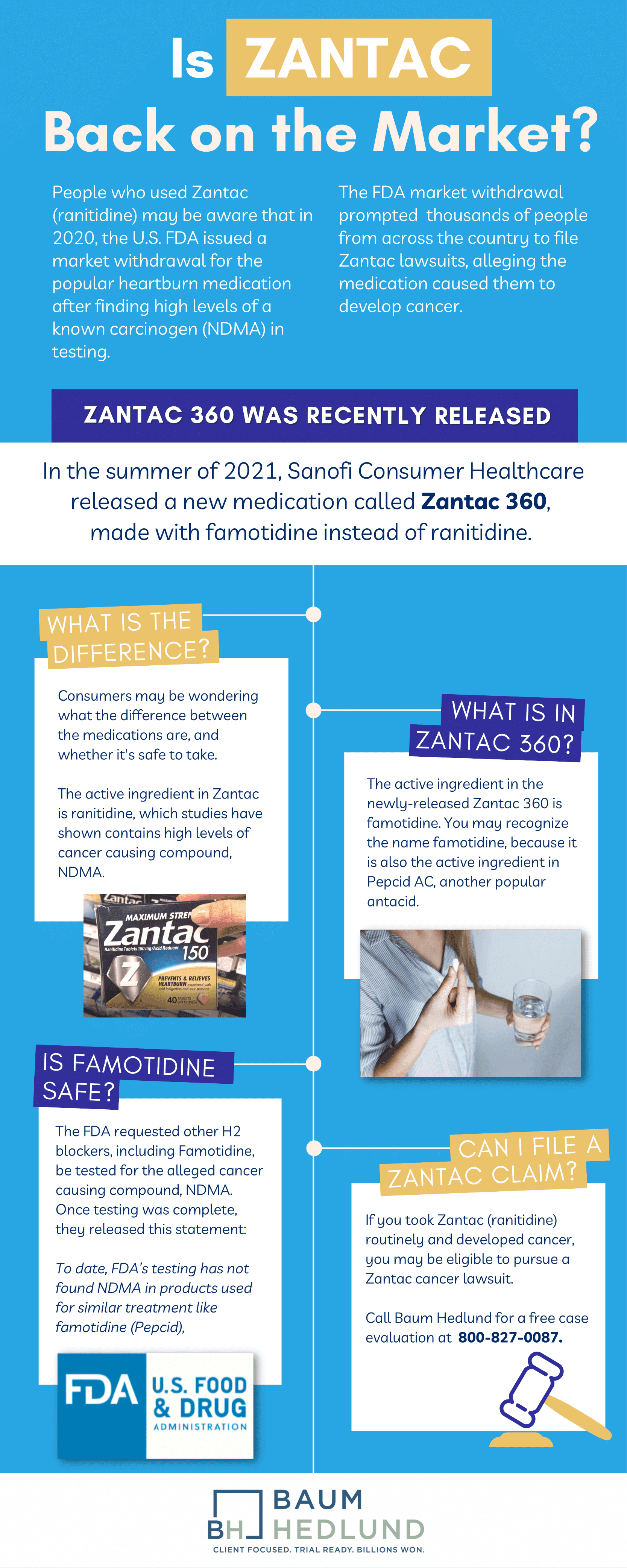
The FDA’s actions prompted many thousands of people from across the country to file Zantac lawsuits alleging the medication caused them to develop prostate cancer, kidney cancer, bladder cancer, breast cancer, and a host of other types of cancer. Yet despite the reported dangers, some people have asked: Will Zantac ever come back on the market?
The answer to that question came in April of 2021…sort of.
Roughly a year after the Zantac market withdrawal announcement, Sanofi Consumer Healthcare U.S. introduced a new Zantac product: Zantac 360, which the company said would hit shelves in the summer of 2021.
However, Zantac 360 is not the same medication as the Zantac that was recalled in 2020. In this blog, we will discuss the newly released Zantac 360, how the drug differs from Zantac, and what the FDA has said about Zantac 360’s safety profile.
What is the New Zantac 360?
Zantac 360 (famotidine) is available over-the-counter (OTC) and comes in two different dosages: 10mg and 20mg oral tablets. The medication, which has been available in the U.S. since around June of 2021, is currently recommended for adults and children over the age of 12. People can take Zantac 360 15 minutes before eating or drinking to help prevent heartburn or after symptoms develop to relieve heartburn.
Famotidine belongs to the drug class known as histamine H2-receptor antagonists (or simply H2 blockers), which decrease the amount of acid produced by the stomach. Ranitidine is also an H2 blocker. With ranitidine pulled from the shelves, the only other H2 blocker currently on the market is Tagamet HB (cimetidine).
What is the Difference Between Zantac and Zantac 360?
The active ingredient in Zantac is ranitidine. Numerous studies have shown that ranitidine contains high levels of N-Nitrosodimethylamine (NDMA) and increases the risk of developing several types of cancer. The FDA, the Environmental Protection Agency (EPA), and the World Health Organization (WHO) all classify NDMA as a carcinogen (capable of causing cancer).
The active ingredient in the newly-released Zantac 360 is famotidine. You may recognize famotidine because it is also the active ingredient in brand name Pepcid AC, another popular antacid. Zantac 360 has no distinct treatment advantages over Pepcid as they both use the same active ingredient and provide the same relief from heartburn.
Is Famotidine Safe?
Following the Zantac recall announcements, the FDA requested that other H2 blockers, including famotidine, be tested for NDMA. After evaluating famotidine and several other heartburn medications, the FDA issued a statement (current as of April 1, 2020), which included the following:
To date, FDA’s testing has not found NDMA in products used for similar treatment like famotidine (Pepcid), cimetidine (Tagamet), esomeprazole (Nexium), lansoprazole (Prevacid) or omeprazole (Prilosec).
Famotidine Side Effects
Famotidine does come with some side effects. The most common include:
- Headache
- Dizziness
- Constipation
- Diarrhea
Can I File a Zantac Case?
If you took Zantac (ranitidine) routinely and developed any of the following cancers, you may be eligible to pursue a Zantac cancer lawsuit:
- Bladder cancer
- Breast cancer
- Esophageal cancer
- Kidney cancer
- Liver cancer
- Lung cancer (for non-smokers)
- Pancreatic cancer
- Prostate cancer
- Stomach cancer
- Thyroid cancer
Zantac lawyers from the national law firm of Baum Hedlund Aristei & Goldman represent thousands of people from all across the country in cases against the drug manufacturers. According to vice president and senior shareholder R. Brent Wisner, the Zantac litigation has the potential to “dwarf” what the firm achieved in the Roundup cancer litigation.
We are currently offering free and confidential case evaluations for those who are interested in pursuing a claim.
Contact us or call (855) 948-5098 today to speak with our legal team about your case.


