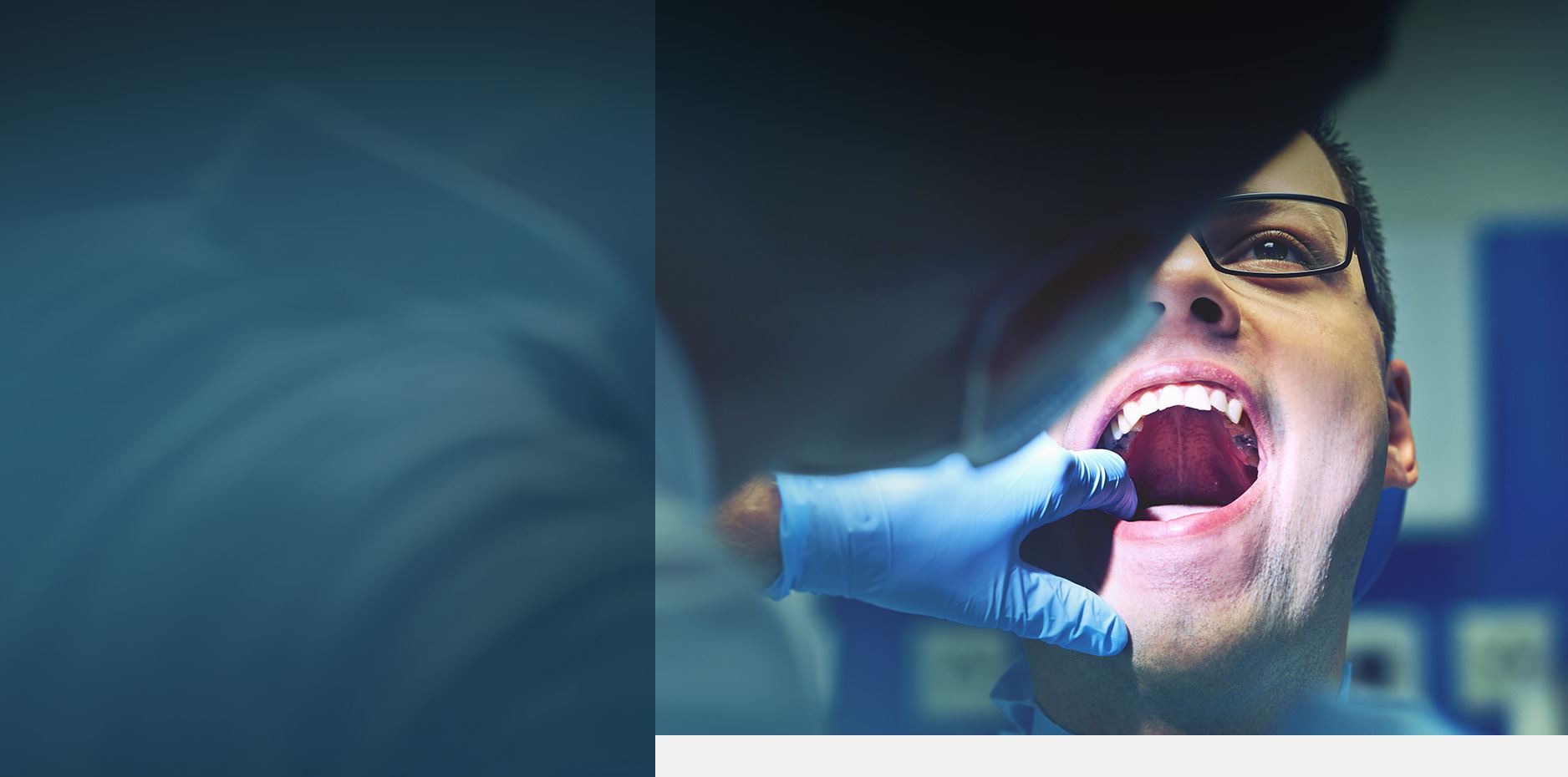
Suboxone Tooth Decay Lawsuit
People across the nation are filing Suboxone tooth decay lawsuits alleging drug maker Indivior and other defendants knew Suboxone causes tooth decay and other dental injuries but failed to warn consumers and the medical community of the risks.
A combination of buprenorphine and naloxone, Suboxone helps people addicted to opioids avoid withdrawal symptoms. But studies have shown that Suboxone can cause significant tooth decay, potentially leading to tooth loss, fractures, and other dental pain.
Suboxone lawyers are bringing tooth decay lawsuits on behalf of individuals alleging Indivior and other defendants knew about significant dental problems associated with Suboxone use for many years but took no steps to alert patients or prescribers of the known dangers.
Per Suboxone lawsuits, patients only know about the link between Suboxone and tooth decay because the U.S. Food and Drug Administration (FDA) issued a Drug Safety Communication in 2022 “warning that dental problems have been reported with medicines containing buprenorphine that are dissolved in the mouth. The dental problems, including tooth decay, cavities, oral infections, and loss of teeth, can be serious and have been reported even in patients with no history of dental issues.” Prior to the FDA Suboxone warning, the drug manufacturers took no action to warn patients of the known risks, the lawsuits say.
If you used Suboxone for at least six months prior to 2022 and developed problems with your teeth—including tooth decay, broken teeth, extractions, root canals, or other injuries—you may qualify for a Suboxone lawsuit. Please fill out our contact form below. If you have any questions and would like to speak with our legal team, please give us a call at 855-948-5098.
CAN I STILL APPLY FOR SUBOXONE LAWSUIT?
Yes, you can still qualify for the Suboxone lawsuit if you meet certain requirements. In this video, attorney Bijan Esfandiari talks about the lawsuit allegations as well as who may be eligible for a Suboxone payout.
Suboxone Lawsuit Update
June 11, 2024: Over 350 suboxone lawsuits are consolidated in the federal MDL. While the litigation continues to grow with more cases filed monthly, there have not yet been any publicly disclosed suboxone settlement agreements for tooth decay. No trial dates have been scheduled.
- Numerous individual claims consolidated to promote efficiency and collective resolution.
- One or more individuals to represent an entire group's legal interests.
- Shared discovery (evidence is collected and shared among all the plaintiffs, reducing redundancy and increasing efficiency).
However, MDLs and class actions are different in the following ways:
- In a class action, many people with similar claims are represented by one or more individuals (class representatives). In an MDL, individual claims are maintained, but pretrial procedures are coordinated among similar cases.
- In a class action, potential plaintiffs are automatically included as part of the class unless they opt-out. In an MDL, plaintiffs initiate their own lawsuits and opt-in to be part of the coordinated proceedings.
- Most importantly, in a class action, there is one judgment for all class members. In an MDL, each plaintiff maintains control over their individual case, with outcomes determined separately.
Class action attorneys at Wisner Baum believe the JPML will issue a ruling in February to consolidate cases into a Suboxone MDL.
January 12, 2024: A new lawsuit was filed today in Pennsylvania alleging Suboxone film caused teeth damage, which resulted in extensive dental procedures. The plaintiff is seeking damages against the defendants for failing to warn about the potential for dental erosion and tooth decay associated with Suboxone film.
December 15, 2023: The defendants in the Suboxone tooth decay lawsuits recently filed a response to the request for multidistrict litigation (MDL) consolidation. Suboxone defendants agreed with the motion to create a new Suboxone MDL and requested that consolidated cases be assigned to Judge Philip Calabrese in the Northern District of Ohio. Attorneys believe a Suboxone MDL will be established in early 2024.
November 28, 2023: Suboxone lawyers representing the plaintiffs in tooth decay lawsuits have petitioned the U.S. Judicial Panel on Multidistrict Litigation (JPML) to consolidate all federal lawsuits related to Suboxone into a single multidistrict litigation (MDL). The formation of an MDL, which shares similarities with a class action lawsuit, would consolidate cases in front of one judge and provide a more efficient way to handle the litigation.
November 5, 2023: More than a dozen new Suboxone lawsuits were filed in federal courts against Indivior over the last two weeks. Eight of the complaints were filed in the Northern District of Ohio, which currently has the most pending Suboxone lawsuits in the country.
October 25, 2023: The maker of Suboxone (Indivior) agreed to pay a settlement worth $385 million to resolve antitrust claims. This settlement has no effect on Suboxone lawsuits alleging tooth decay. Instead, Indivior agreed to settle claims that accused the company of illegally suppressing generic competition.
October 16, 2023: An Ohio man filed a Suboxone tooth decay lawsuit in federal court. The plaintiff alleges he became addicted to opioids after receiving a script from his doctor and was eventually prescribed Suboxone to treat his addiction. After a year and four months of taking Suboxone, the plaintiff alleges he suffered severe tooth decay and required several tooth extractions. According to the Suboxone lawsuit, neither the plaintiff nor his doctor knew that Suboxone causes tooth decay.
April 14, 2023: The Federal Trade Commission (FTC) paid some consumers who did not join the Suboxone class action lawsuit before the original deadline.
Suboxone is a drug approved by the FDA to help treat Opioid Use Disorder (OUD). It is made up of two medications, buprenorphine and naloxone. Buprenorphine works by blocking the opioid receptors in the brain, which can reduce withdrawal symptoms and cravings for opioids when used under medical supervision.
Naloxone (Narcan) is included in Suboxone to help reverse the dangerous symptoms of an opioid overdose and make it harder for the medication to be abused.
Suboxone is also available in generic forms.
Suboxone Tablets and Film Strips
Suboxone comes in the form of sublingual films or tablets, which are administered as part of a comprehensive treatment program that includes counseling and therapy. The method of taking Suboxone is through oral administration. Suboxone is available in three forms:
1. Sublingual Suboxone Tablets (most commonly round, orange, uncoated): These tablets are placed underneath the tongue.
2. Sublingual Suboxone Film Strips (most commonly paper thin, orange rectangle or square): These film strips are also placed underneath the tongue.
3. Buccal Suboxone Film Strips (most commonly paper thin, orange rectangle or square): These film strips are placed between the gums and teeth.
Suboxone is most commonly offered in four different dosages:
- 2 mg buprenorphine and 0.5 mg naloxone
- 4 mg buprenorphine and 1 mg naloxone
- 8 mg buprenorphine and 2 mg naloxone
- 12 mg buprenorphine and 3 mg naloxone
What is Belbuca?
Belbuca contains the same active ingredient as Suboxone (buprenorphine) but does not include naloxone (Narcan). Belbuca is not approved for OUD; it is instead prescribed to patients with chronic pain. There are no generic versions of Belbuca.
Belbuca Dosage
Belbuca is available in many dosages as a square yellow dissolvable film:
- 75 mcg
- 150 mcg
- 300 mcg
- 450 mcg
- 600 mcg
- 750 mcg
- 900 mcg
What is Subutex?
Like Belbuca, Subutex contains only buprenorphine. Reckitt Benckiser (now Indivior) discontinued Subutex in the U.S. in 2011 after developing new formulations that were less likely to be abused. Subutex is still available in generic forms, but practitioners most often prescribe Suboxone instead.
Does Suboxone Cause Tooth Decay?
Yes, according to the medical literature. In December of 2022, a research letter published in the Journal of the American Medical Association reported dental adverse events associated with buprenorphine medications like Suboxone. The study, which examined thousands of patients from 2006–2020, found “an increase in the risk of adverse dental outcomes associated with sublingual buprenorphine/naloxone…”
While this study is the most robust to date in terms of patient cohort and duration, it is not the only research confirming a link between Suboxone and problems with teeth. In 2012, Harvard Medical School professors affiliated with Brigham and Women’s Hospital in Boston published a case report on a patient with sudden dental problems while using Suboxone tablets. The authors concluded that the “patient’s experience of a sudden decline in her oral health without any changes in her dental hygiene practices or sugary food/beverage consumption raises the possibility that chronic use of sublingual buprenorphine/naloxone may have played a role.”
A year later, the lead author from the 2012 study worked with Harvard colleagues to publish a case series of eleven patients at Brigham and Women’s Hospital. The study patients experienced dental caries, dental fillings, cracked teeth, crown replacements, root canals, and tooth extractions. The authors noted that cavities and tooth erosion “occur when teeth are exposed to an environment that has low pH.”
pH is a scale running between 0 and 14 measuring the acidity or basicity of an aqueous solution. It inversely indicates the activity of hydrogen ions in the solution. A pH of 7 indicates neutral. A pH of less than 7 indicates acidity and a pH of greater than 7 indicates a base.
A Suboxone tablet measures acidic at 3.4 pH. Patients are specifically instructed to keep the Suboxone tablet and the accumulating saliva in their oral cavity to maximize absorption. Based on the average ingestion of Suboxone tablets three times daily for an average span of nine minutes to dissolve, the authors of the 2013 and 2012 studies concluded that “prolonged contact between tooth surfaces with buprenorphine/naloxone, therefore, may be a contributing factor in the alteration of the tooth microbial profile and/or the pH to promote dental caries, similar to what has been previously reported in patients who use methamphetamine.”
How Does Suboxone Affect Your Teeth?
Suboxone is acidic to maximize buprenorphine absorption while minimizing absorption of the naloxone. This acidic formulation leads to dental erosion and decay.
Suboxone teeth problems include:
- Tooth decay
- Tooth loss
- Extraction
- Oral infections
- Cracked teeth
- Cavities
- Root canal
- Dental caries (loss of enamel)
- Crown or crown replacement
- Gingivitis and other gum disease
One thing to remember is that these dental issues are not limited to Suboxone; the FDA also warned about Subutex and Belbuca teeth issues.
Can I Sue Suboxone for Ruining My Teeth?
Yes, you can pursue compensation in a Suboxone lawsuit if you meet certain requirements (more on this below). Even if you had problems with your teeth before using Suboxone, Belbuca or Subutex, lawsuits allege the medication increased your risk of tooth decay and other dental issues.
how to join Suboxone Lawsuit
The only way to know for certain if you qualify for a Suboxone lawsuit is to fill out a case evaluation form. A Suboxone lawyer will review your case and determine if you are eligible.
Potential plaintiffs in Suboxone lawsuits are individuals who used Suboxone for at least six months prior to 2022 and later experienced severe tooth decay or loss, necessitating corrective dental procedures such as fillings, extractions, or implants.
Please keep in mind that the criteria to qualify for a Suboxone lawsuit may change over time due to court rulings, scientific findings, and other variables.
Who Are the Suboxone Lawsuits Against?
Indivior, Inc. and Aquestive Therapeutics Inc. are the pharmaceutical companies named as defendants in the Suboxone lawsuits. Indivior, specifically, is the primary manufacturer of the drug, but both companies play a significant role in the production and marketing of Suboxone.
Indivior was originally a division of Reckitt Benckiser, a larger pharmaceutical company based in the United Kingdom. When the market for opioid dependency drugs expanded, Reckitt spun off Indivior as a separate publicly traded company. Indivior's close relationship with Reckitt, which continues to own a significant percentage of shares, adds weight to their involvement in lawsuits against Suboxone.
It should be noted that Indivior has already faced legal action stemming from its activities in marketing and selling Suboxone.
In October of 2023, Indivior paid a $385 million settlement resolving lawsuits brought by drug wholesalers alleging the company illegally suppressed generic competition for Suboxone.
In August of 2023, Indivior paid a similar settlement of $30 million to resolve a class action lawsuit brought by health care plans.
The Department of Justice indicted the company in June of 2023 for participating in an illegal scheme that funneled patients to specific doctors prescribing Suboxone. To resolve the matter, Indivior agreed to pay a settlement of $102 million to 41 states and Washington D.C.
In 2021, the Federal Trade Commission announced a $60 million settlement with Indivior over deceptive marketing allegations. Nearly 52,000 people received a payout as part of the settlement.
Is There a Suboxone Class Action Lawsuit?
Suboxone tooth decay lawsuits are filed for individuals; they are not part of a class action. While these lawsuits make similar allegations against the same defendants, the plaintiffs have differing claims for damages.
How do the courts handle these types of cases? Through the creation of a multidistrict litigation (MDL). Although not classified as a typical class action lawsuit, an MDL consolidates all federal cases for efficient pretrial proceedings. This allows plaintiffs with similar allegations against the defendants to collaborate and present a stronger collective argument.
In February 2024, theJudicial Panel on Multidistrict Litigation (JPML) issued an order to consolidate Suboxone teeth decay lawsuits into an MDL. The order consolidates claims filed in several federal courts before the Hon. Judge J. Philip Calabrese for the U.S. District Court in the Northern District of Ohio.
is there a suboxone settlement?
No, there has not been a Suboxone settlement. With the formation of the Suboxone MDL, we anticipate litigation to last through 2024. In mass torts, settlement negotiations could begin at any time, but typically, they do not progress until bellwether trials are due to begin, and we are several months away from trial dates.
Call Now to See If You Have a Lawsuit Against Suboxone
Wisner Baum offers free case evaluations for those who may have suffered Suboxone teeth problems. Please fill out our case evaluation form or give us a call at 855-948-5098
Our Case Results

-
$10 Million Settlement A Major Foreign Plane Crash
Wisner Baum obtained a $10 million settlement for the death of a passenger in a major foreign plane crash.
-
$14 Million Settlement A Major US Plane Crash
Wisner Baum obtained a $14 million settlement for the death of a passenger in a major US plane crash.
-
$17.5 Million Settlement A Major US Plane Crash
Wisner Baum obtained a $17.5 million settlement on behalf of a client who was killed in a major U.S. plane crash.
-
$10 Million Settlement Celexa-Lexapro Pediatric Class Action
$10 million pediatric class action re false promotion of Celexa and Lexapro. Babies born to women who have used Lexapro and other similar medications such as Zoloft, Celexa, Prozac, Paxil, and Symbyax are at an increased risk for birth defects.
-
$8.5 Million Verdict Commercial Truck Accident
Wisner Baum secured a $8.5 million wrongful death verdict against the food industry company, Tyson Foods, for the wrongful death of a young man.
-
$28 Million Settlement Defective Drug Class Action
$28 million Paxil defective drug class action. A class action has been brought in the US territory of Puerto Rico against UK-based drug major GlaxoSmithKline.

Client-Focused Representation
REVIEWS & TESTIMONIALS
We believe our track record speaks for itself. But you don’t have to take our word for it. See what our clients have to say about working with us.
-
"I Can’t Imagine a Better Law Firm"
Multiple lawyers recommended Wisner Baum to me and I have been consistently impressed with the quality of their work.
- Best Law Firms Survey -
"They Are About Changing the Systems..."
Wisner Baum are not only amazing attorneys but more importantly, they are activists. They are about changing the systems which got us into trouble in the first place. They understand their role in the process of making change.
- Kim Witczak -
"Top Legal Minds in the Country"
The Wisner Baum firm has some of the top legal minds in the country; they are driven, determined, trustworthy, ethical and passionate.
- From Best Lawyers® Best Law Firms





