Celexa Information and Birth Defects
We are no longer accepting new Celexa birth defect cases
Citalopram, marketed as Celexa, is a selective serotonin reuptake inhibitor (SSRI) antidepressant used to treat major depression and is often prescribed off-label to treat a wide variety of conditions. Originally created in 1989 by the pharmaceutical company Lundbeck, Celexa is an SSRI antidepressant that is occasionally used to treat attention deficit hyperactivity disorder (ADHD), obsessive compulsive disorder (OCD), and premenstrual dysphoric disorder (PMDD).
Today, Forest Laboratories manufactures and markets Celexa, along with a very similar and a more potent SSRI antidepressant called Lexapro. Celexa (citalopram) and Lexapro (escitalopram) share a very similar pharmacological make-up.
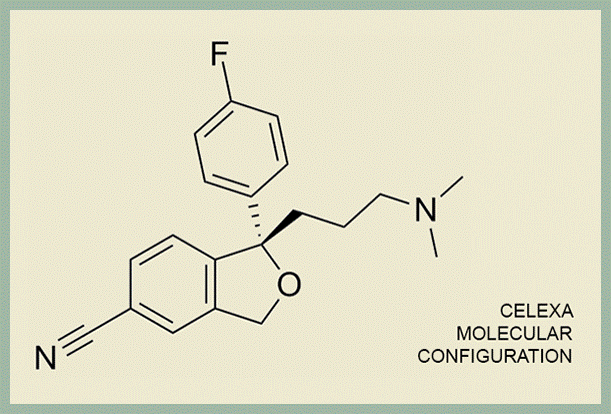
How are Celexa and Lexapro similar? Imagine the molecular configuration below printed off on a piece of paper pressed against a mirror—Celexa is made of both the paper and the mirror image, and Lexapro is just the mirror image. Or imagine two hands pressed together—Celexa is both hands and Lexapro is just the left hand.

Detailed Celexa Information
Pharmaceutical: Celexa
Manufacturer: Forest Laboratories
Generic name: citalopram
Drug Class: Selective serotonin reuptake inhibitor (SSRI)
CAS Number: 59729-33-8
Guidelines:
Celexa (citalopram) is used to treat symptoms associated with major depression. The drug is administered orally in the form of a tablet. Warning:the Food and Drug Administration (FDA) has warned physicians against prescribing more than 40 milligrams of citalopram per day due to a risk of changing the electrical activity of the heart, which can be fatal.
Contraindications:
- Celexa is contraindicated for people taking a monoamine oxidase inhibitor (MAOI). Use of MAOIs within two weeks of Celexa treatment can cause serotonin syndrome.
- Celexa should also not be taken with St. John’s Wort, tryptophan.
Adverse outcomes:
- drowsiness
- weight changes
- fatigue, insomnia
- nausea
- vivid dreaming
- decreased libido
- frequent urination
- dry mouth
- anorgasmia
- sweating
- increased anxiety
- mood swings
- bruxism
- vomiting
- cardiac arrhythmia
- dizziness
- headaches
- blood pressure fluctuation
- dilated pupils
- hallucinations
- increased trembling and excessive yawning
- convulsions
Serious Adverse Outcome:
Suicide and serotonin syndrome
Warnings:
- Do not take Celexa if you are allergic to active or inactive ingredients in the drug.
- Do not take Celexa if you are taking an MAOI.
- Allow two weeks after ending an MAOI before starting Celexa treatment.
- Likewise, allow two weeks after ending Celexa treatment before starting an MAOI.
Medical Studies:
- New England Journal of Medicine Study:Selective Serotonin-Reuptake Inhibitors and Risk of Persistent Pulmonary Hypertension of the Newborn
- New England Journal of Medicine Study:First-Trimester Use of Selective Serotonin-Reuptake Inhibitors and the Risk of Birth Defects
Pregnancy Category:
The FDA classifies Celexa as a Pregnancy Category C drug. This means that even though the drug has not been studied in pregnant women, animal studies have demonstrated an increased risk to a fetus if Celexa is taken during pregnancy.
Our Case Results

-
$10 Million Settlement A Major Foreign Plane Crash
Wisner Baum obtained a $10 million settlement for the death of a passenger in a major foreign plane crash.
-
$14 Million Settlement A Major US Plane Crash
Wisner Baum obtained a $14 million settlement for the death of a passenger in a major US plane crash.
-
$17.5 Million Settlement A Major US Plane Crash
Wisner Baum obtained a $17.5 million settlement on behalf of a client who was killed in a major U.S. plane crash.
-
$10 Million Settlement Celexa-Lexapro Pediatric Class Action
$10 million pediatric class action re false promotion of Celexa and Lexapro. Babies born to women who have used Lexapro and other similar medications such as Zoloft, Celexa, Prozac, Paxil, and Symbyax are at an increased risk for birth defects.
-
$8.5 Million Verdict Commercial Truck Accident
Wisner Baum secured a $8.5 million wrongful death verdict against the food industry company, Tyson Foods, for the wrongful death of a young man.
-
$28 Million Settlement Defective Drug Class Action
$28 million Paxil defective drug class action. A class action has been brought in the US territory of Puerto Rico against UK-based drug major GlaxoSmithKline.

Client-Focused Representation
REVIEWS & TESTIMONIALS
We believe our track record speaks for itself. But you don’t have to take our word for it. See what our clients have to say about working with us.
-
"I Can’t Imagine a Better Law Firm"
Multiple lawyers recommended Wisner Baum to me and I have been consistently impressed with the quality of their work.
- Best Law Firms Survey -
"They Are About Changing the Systems..."
Wisner Baum are not only amazing attorneys but more importantly, they are activists. They are about changing the systems which got us into trouble in the first place. They understand their role in the process of making change.
- Kim Witczak -
"Top Legal Minds in the Country"
The Wisner Baum firm has some of the top legal minds in the country; they are driven, determined, trustworthy, ethical and passionate.
- From Best Lawyers® Best Law Firms





