
IVC Filter Complications
The apparently endless variety of IVC filter complications stem from the role that the inferior vena cava plays in the circulatory system and its location in the body relative to several critical organs.
IVC filter complications stem from one of three major types of filter malfunctions:
- Migration of the filter or a broken filter strut.
- Filter penetration of the inferior vena cava.
- Blockage or occlusion of the filter.
IVC Filter Migration
The inferior vena cava is a large vein that carries deoxygenated blood from the lower body to the heart. From there, the heart pumps the blood through the pulmonary arteries to both lungs. So if an IVC filter moves or fractures, the filter or one of its broken struts (the legs that hold the filter to the walls of the IVC) may be carried to the heart (right atrium and right ventricle), pulmonary arteries or lungs and lodge itself there.
This process is known as migration or embolization—a medical term for the movement of a mass through a blood vessel to lodge somewhere else in the body.
Many of the most severe IVC filter complications involve migration of the filter or the embolization of one or more filter struts that have fractured and separated from the device. In December 2013, the European Journal of Vascular and Endovascular Surgery published an article on the death of a 60-year old man as a result of the embolization of an IVC filter to the patient’s right atrium.
The March 2015 issue of the Journal of the American College of Cardiology describes the case of 64-year old may who was admitted to the hospital complaining of chest pain. A filter strut had embolized to the right ventricle and punctured the pericardium (the sac that encloses the heart), causing it to fill with fluid. Heart surgery was required.
Penetration of the Inferior Vena Cava
The inferior vena cava is close to the spine and several other internal organs.
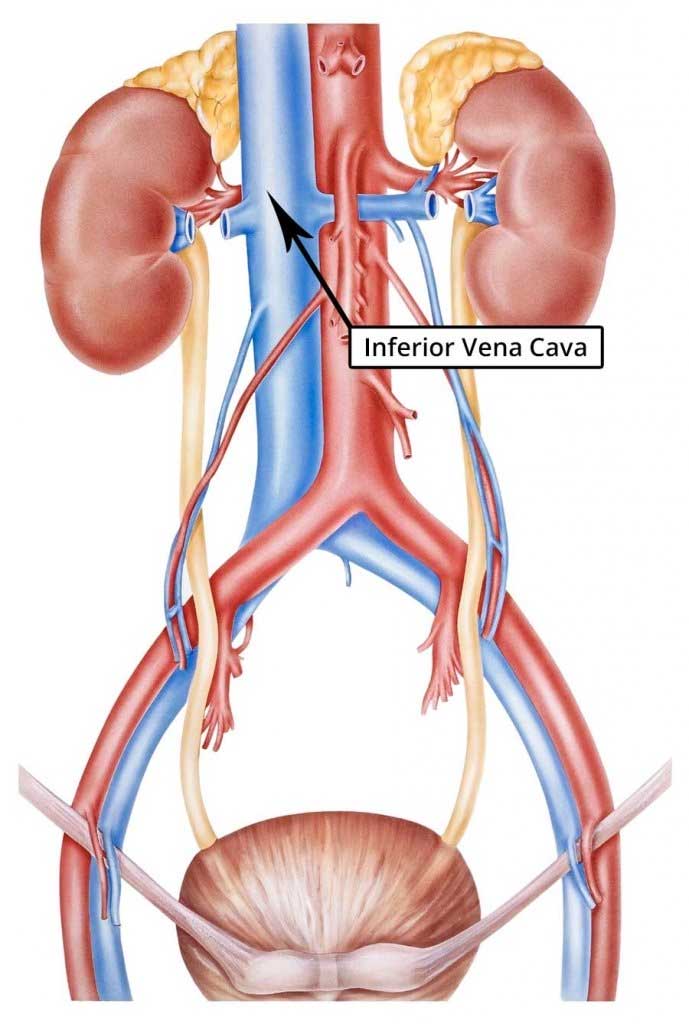
This is important because the IVC filter struts frequently penetrate the walls of the inferior vena cava and come into contact with internal organs near the vein.
A 2009 review of the medical literature published in the International Journal of Angiology found that penetration of the inferior vena cava has been linked to injuries of the:
- Duodenum – the first segment of the small intestine
- Aorta – the body’s main artery that carries oxygenated blood to the body
- Portal vein – a vein that carries blood from the stomach, intestines, pancreas and spleen to the liver
- Small and large intestine
- Pancreas
- Kidney
- Renal vein – the veins that carry purified blood from the kidney to the inferior vena cava
- Spinal column
- Diaphragm
- Genital and urinary system
If you or a loved one has been injured by an IVC filter, or is under threat of injury, the IVC filter team at Wisner Baum would like to hear from you. Call (855) 948-5098 today.
IVC Filter Complications – Filter Occlusion
In addition to the problem of filter migration, embolization of the struts and penetration of the IVC, there is an additional complication: the blockage or occlusion of the filter due to blood clots being captured by the filter or forming as a result of filter placement. According to a February 2013 study in Vascular Medicine, placement of an IVC filter may promote the formation of blood clots. “Occlusion of IVC filters remains their most frequent complication,” the authors wrote.
IVC Filter Complications – The MAUDE Database
One of the best sources of information about IVC filter complications is within a database maintained by the U.S. Food and Drug Administration known as the Manufacturer and User Facility Device Experience (MAUDE) database. Researchers use this database to study problems associated with the filters.
Federal law requires manufacturers and importers of medical devices, as well as medical facilities (such as hospitals, ambulatory surgical centers, nursing homes and outpatient clinics), to let U.S. Food and Drug Administration (FDA) know when they have learned of significant problems associated with a medical device.
They must file reports and those reports, along with the information contained in them, are then stored in the MAUDE database. Sometimes voluntary reports are also filed by health care professionals and consumers.
Perhaps the most important element to realize about the reports in the MAUDE database is that each report involves either death, serious injury or malfunctions that “would be likely to cause or contribute to a death or serious injury” if these defects were to occur again.
“He allegedly experienced worsening pain and discomfort as fragments of the filter ‘became embedded in the tissue throughout his body.’ Decedent allegedly took his own life…after being ‘unable to find relief from constant pain.’” -From a MAUDE database report submitted to the FDA on October 22, 1015.
MAUDE Database in Medical Research
In 2014, doctors in Northwestern University’s Department of Radiology published the results of their investigation into IVC filter incident reports contained in the MAUDE database. They searched the database for adverse events that had been reported between January 2009 and December 2012 and found 1,606 such events involving 1,057 IVC filters. 1,394 events involved retrievable filters, those that are meant to be removed as soon as there is no longer a need, and 212 were tied to permanent filters. Not surprisingly, the filing of an IVC filter lawsuit is most often tied to the retrievable type filters.
Out of 1,606 adverse events reported, the researchers found the following complications:
| Complication | Explanation | Number of occurrences |
| Fracture | Breaking of one of the limbs that hold the filter in place | 350 |
| Migration | Movement of the filter after placement. This may be a small distance or the filter may travel all the way to the heart. | 215 |
| Limb embolization | Embolization refers to the travel of broken limbs through the vein to other locations (often the heart). | 154 |
| Tilt | Tilting of the filter in the inferior vena cava | 197 |
| IVC penetration | Penetration of the IVC by one or more limbs. The limbs may completely penetrate the IVC and pierce external organs. | 228 |
| VTE/PE | VTE – Venous thromboembolism: the formation of blood clots in the vein. PE – Pulmonary embolism: blockage of a lung artery, usually by a blood clot that travels from a vein in the leg. | 30 |
| IVC thrombus | Formation of a blood clot in the IVC | 41 |
| Placement malfunctions | 318 | |
| Other | 73 |
As mentioned above, the limbs of the IVC filter may pierce through the inferior vena cava and come into contact with nearby organs. The Northwestern doctors found 28 cases in which the device had penetrated into the duodenum or small bowel, 14 cases of penetration into the aorta, and 12 cases of penetration into structures adjacent to the IVC, including vertebral bodies and disks—nine producing bleeding.
A recent IVC filter lawsuit has alleged (backed by autopsy findings) that the filter pierced the inferior vena cava, causing internal bleeding which led to a woman’s death.
In another IVC filter lawsuit, a Mississippi man alleges that the filter perforated the inferior vena cava and was leaning against the abdominal aorta—the blood vessel that supplies oxygenated blood to the organs around the abdomen and pelvis, as well as to the legs. The position of the device made it too dangerous to remove.
MAUDE database report submitted to the FDA on November 3, 2015:
“Event description: It was reported that approximately five years post vena cava filter deployment; an inferior vena cava gram performed allegedly demonstrated a detached limb in the right kidney, with two filter limbs penetrating the IVC wall. There was no report of an attempt to retrieve the filter or detached limb was made. Approximately six years post vena cava filter deployment an open surgical procedure was performed to remove the filter; reportedly four filter limbs had perforated the IVC wall. The filter was removed successfully….possible complications include, but are not limited to, the following: perforation or other acute or chronic damage of the IVC wall…. All of the above complications have been associated with serious adverse events such as medical intervention and/or death.”
IVC Filter Complications and Filter Brands
Information in the database suggests that certain IVC filter complications are more closely associated with one brand than another, perhaps due to filter design. The Northwestern University study authors found that fracture of the filter device was most commonly reported in Bard filters. They also noted that filter fracture and migration were cited as reasons the Bard Recovery and G2 filters were removed from the market. Penetration of the IVC by the filter limbs was most common with the Cook Celect filter, which has been reported in the medical literature to have a rate of IVC penetration of 86.1%. Placement malfunctions were associated more with the OPTEASE and Cook Günther Tulip filters.
If you or a loved one has been injured by an IVC filter, or is under threat of injury, the IVC filter team at Wisner Baumwould like to hear from you. Please contact our firm for a free consultation.
Our Case Results

-
$265 Million Settlement Fatal Train Crash
In 2016, Wisner Baum attorney Timothy A. Loranger and six other attorneys in the Plaintiffs’ Management Committee were able to secure a $265 million settlement for victims of the 2015 Amtrak 188 derailment in Philadelphia, one of the largest in the U.S. for 2016.
-
$14 Million Settlement A Major US Plane Crash
Wisner Baum obtained a $14 million settlement for the death of a passenger in a major US plane crash.
-
$12 Million Settlement Helicopter Crash
Wisner Baum secured a $12 million settlement for a passenger who was injured in a helicopter crash.
-
$10 Million Settlement A Major Foreign Plane Crash
Wisner Baum obtained a $10 million settlement for the death of a passenger in a major foreign plane crash.
-
$2.0 Billion Verdict Personal Injury
In May of 2019, the jury in the case of Pilliod et al. v, Monsanto Company ordered the agrochemical giant to pay $2.055 billion in damages to the plaintiffs, Alva and Alberta Pilliod, a Bay Area couple in their 70s.
-
$80 Million Verdict Personal Injury
Wisner Baum attorneys served on the trial team in the case of Hardeman v. Monsanto Company, which resulted in an $80 million jury verdict for the plaintiff, Edwin Hardeman.

Client-Focused Representation
REVIEWS & TESTIMONIALS
We believe our track record speaks for itself. But you don’t have to take our word for it. See what our clients have to say about working with us.
-
"I Can’t Imagine a Better Law Firm"
Multiple lawyers recommended Wisner Baum to me and I have been consistently impressed with the quality of their work.
- Best Law Firms Survey -
"They Are About Changing the Systems..."
Wisner Baum are not only amazing attorneys but more importantly, they are activists. They are about changing the systems which got us into trouble in the first place. They understand their role in the process of making change.
- Kim Witczak -
"Top Legal Minds in the Country"
The Wisner Baum firm has some of the top legal minds in the country; they are driven, determined, trustworthy, ethical and passionate.
- From Best Lawyers® Best Law Firms





