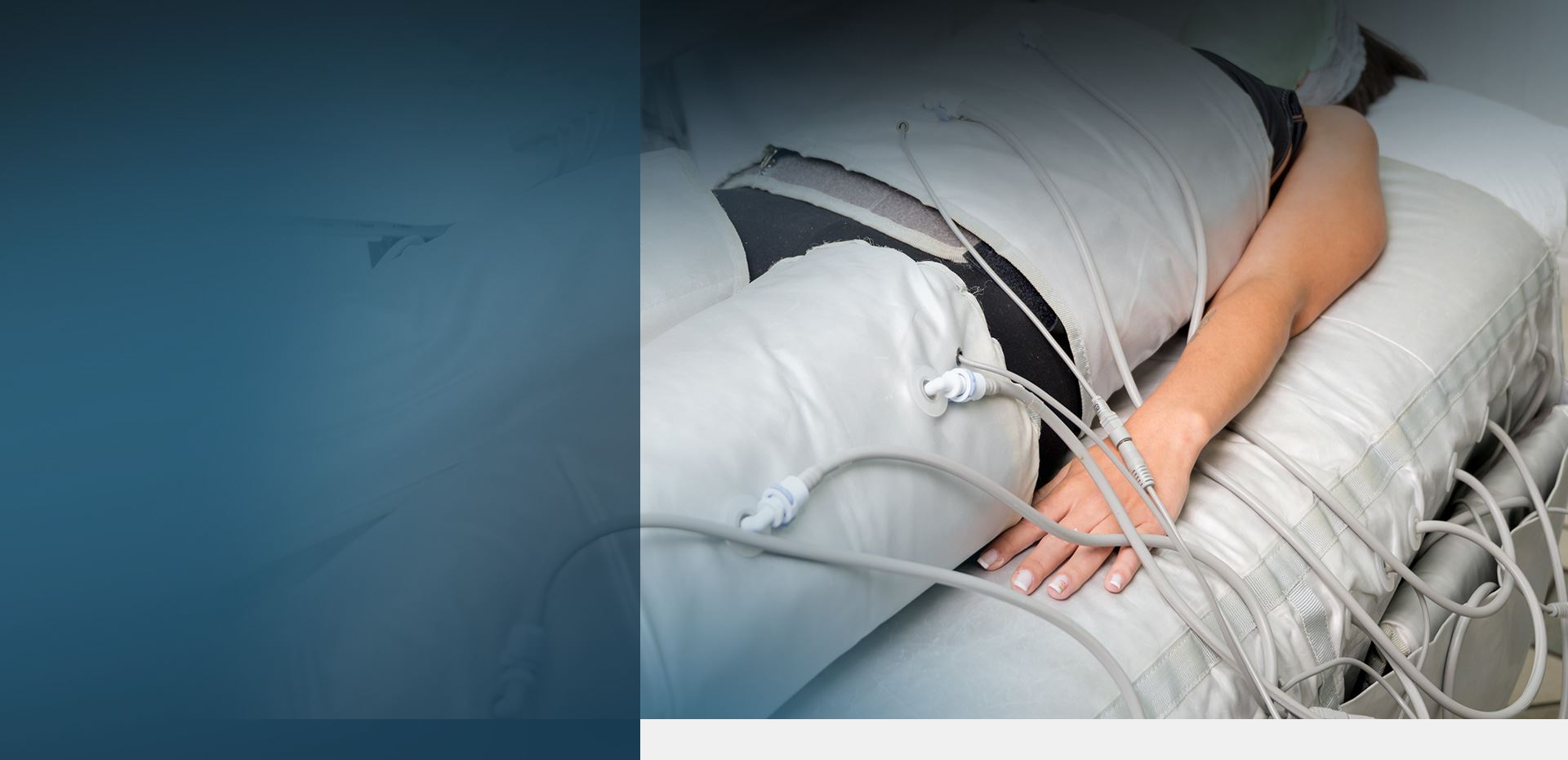
Electroconvulsive Shock “Therapy” (ECT) Lawsuit
What You Should Know About ECT
- ECT medical devices are not approved by the U.S. Food and Drug Administration.
- There are no long-term studies that show ECT is safe or effective.
- ECT can cause brain damage, permanent memory loss and neurocognitive injury.
- Survivors have called ECT treatment “barbaric” and “torture.”
- Wisner Baum served as lead counsel in the trial of Jeffrey Thelen v. Somatics, LLC and Elektrika, Inc. Mr. Thelen alleged Somatics negligently failed to adequately warn about the known risks associated with its ECT machines, including brain damage and memory loss. The jury found that Somatics failed to warn about the risks associated with its ECT machines. However, the jury also concluded that this failure was not the legal cause of Mr. Thelen’s injuries. Our firm has filed a motion for a new trial.
- Dozens of lawsuits have been filed across the United States by survivors harmed by the MECTA Spectrum ECT machine and Somatics’ Thymatron machine. (MECTA and Somatics are the only manufacturers of ECT devices in the United States.)
- MECTA filed for bankruptcy in late 2021 and no longer sells the Spectrum machine, but began marketing a new machine in 2020, the “Sigma” machine.
- In 2023, the World Health Organization (WHO) and United Nations Human Rights Office of the High Commissioner issued joint guidance to “protect people against abuses in the use of specific mental health interventions, such as electroconvulsive therapy (ECT)…” The publication noted the “significant controversy” surrounding ECT, which has been banned in some countries. “If permitted, ECT must only be administered with the written or documented, free and informed consent of the person concerned,” the publication states.
When you hear the terms electroconvulsive therapy (ECT) or electroshock therapy, the first thing that probably comes to mind is the film One Flew Over the Cuckoo’s Nest. Patients in the film appear to be brutalized during treatment and afterward sit in the ward drooling with vacant expressions. Most believe that an arcane “therapy” like shock treatment must be a thing of the past.
The truth is that a very small minority in the medical community still accept and strongly advocate for ECT shock therapy as a treatment option for severe depression, bipolar disorder, catatonia, agitation associated with dementia, and other issues, and it is this small minority that keep the procedure alive. It was recently estimated that fewer than 1000 psychiatrists practice ECT in the U.S., out of a national total of approximately 49,000 psychiatrists.
While proponents of ECT argue there have been advancements in the delivery of ECT with anesthesia and muscle relaxants, other than preventing broken bones and teeth or bitten tongues, the procedure continues to deliver a significant amount of electricity to the human brain, resulting in damage.
“[To] put this all in perspective, the amount of electric current that an ECT machine puts through a patient’s head is about 200 times what is considered dangerous for accidental electric shock, approximately 100 times what Tasers, cattle prods, and electric fences use, about the same as what is used for stunning pigs before slaughter, and roughly one-fifth as much as the electric chair. In addition, the amount of voltage applied to the head (460 volts) is about 400 times what is required to damage a single brain cell. Clearly this amount of electricity has the potential to cause injury to the brain.” – Dr. Kenneth Castleman, biomedical electrical engineer and former Senior Scientist at NASA’s Jet Propulsion Laboratory.
While there is no national monitoring in the United States for how many people undergo ECT shock treatment on an annual basis, the ballpark figure touted by the ECT industry is roughly 100,000 patients.
ECT’s introduction is not predicated on research designed to test the machines for safety in the long-term. Since its inception, ECT shock treatment has caused countless adverse events. Comments from electroshock survivors describe the treatment as “barbaric” and “torture.” Even one of the treatment’s founding fathers, Ugo Cerletti, understood the effects ECT had on patients:
When I saw the patient’s reaction I thought to myself: this ought to be abolished! Ever since I have looked forward to the time when another treatment would replace electroshock.
What is ECT?
ECT is an abbreviation for electroconvulsive therapy, also referred to as electroshock therapy, electric shock therapy, or simply shock therapy. These days, ECT is most often used on patients with depression. ECT involves passing sufficient electricity through the brain to intentionally cause a seizure. The amount of electricity required in ECT “therapy” is usually between 70 volts and 120 volts, resulting in roughly 800 milliamps (mA) of direct current passing through the brain either across one temporal lobe (unilateral ECT) or across both temporal lobes (bilateral ECT).
Who Makes Shock Therapy Machines?

 In the U.S., the only two companies that still manufacture ECT devices are Somatics and MECTA. Both are defendants in numerous ECT lawsuits alleging the shock therapy devices caused permanent memory loss, brain injury, and other health issues.
In the U.S., the only two companies that still manufacture ECT devices are Somatics and MECTA. Both are defendants in numerous ECT lawsuits alleging the shock therapy devices caused permanent memory loss, brain injury, and other health issues.
Somatics
ECT Device: Thymatron® System IV - Integrated ECT Instrument
Richard Abrams, M.D. and Conrad Swartz, M.D., formed Somatics, LLC in the 1980s to develop an ECT machine. To this day, Somatics has never obtained approval from the U.S. Food and Drug Administration (“FDA”) to market its ECT machine. Like other ECT device manufacturers, Somatics obtained clearance to sell its “Thymatron” ECT device after representing to the FDA that its device was equivalent to an already existing device.
The difference between FDA approval and “clearance” is significant. The FDA spends approximately 1200 hours reviewing a manufacturers submission of scientific data concerning the safety and efficacy of a proposed medical device prior to officially approving it, but the FDA spends only about 20 hours to “clear” a device that is similar to one on the market prior to 1976 (i.e., the “grandfathering” procedure).
Significantly, the FDA stated in its letter clearing the ECT devices:
“This letter does not in any way denote official FDA approval of your device or labeling. Any representation that creates an impression of official approval of this device … is misleading and constitutes misbranding.” (Misbranding is a legal term which means making a false or misleading representation on the label or in other informational materials, which is a violation of FDA regulations.)
In a court deposition, Abrams testified that his company has never performed any clinical trials, studies, or tests to analyze the long-term side effects associated with ECT because “that’s not our business.”
After settling two ECT lawsuits on the eve of trial in October 2018, Somatics changed its website to state, for the first time:
“ECT may result in anterograde or retrograde amnesia. Such post-treatment amnesia typically dissipates over time; however, incomplete recovery is possible. In rare cases, patients may experience permanent memory loss or permanent brain damage.”
MECTA
ECT devices: SpECTrum®, ∑igma™ (pronounced Sigma)
In 1985, James Fling and the engineering staff at Custom Systems developed the first MECTA electroconvulsive therapy machine in Portland, Oregon.
Like Somatics, MECTA has been named as a defendant in shock therapy lawsuits alleging the company failed to comply with multiple administrative orders by the FDA, has never obtained FDA approval for its ECT devices, and has never maintained a system for the timely investigation, evaluation, and reporting of adverse events.
In a 2005 lawsuit, MECTA could not provide any evidence to demonstrate how ECT works. The company could only indicate that its shock device is designed to cause a grand mal seizure. Beyond that, ECT’s “therapeutic” mechanism is entirely theoretical.
In a deposition, MECTA President and CEO Robin Nicol stated that the company “does not do research” and made a decision to “disregard what it characterized as the minority view of ECT” that ECT “causes brain damage and causes memory loss.” Nicol further indicated that she would be “compassionate,” but not curious to know why victims complained about ECT injuries. “We are not responsible for individual patients…. That is not our responsibility from the FDA perspective or from our perspective as medical-device manufacturers,” Nicol said.
Additionally, Nicol noted that, if she “had information that [MECTA’s] devices weren’t safe, it would not be considered unless the information came from double-blind studies,” an ironic statement given neither MECTA nor Somatics has ever conducted such studies because “that’s not our business” and “we don’t do research.” Meanwhile, the companies have happily reaped millions of dollars in sales of their ECT devices.
According to a Citizen Petition to the FDA submitted in 2016, “MECTA’s President’s view was that if patients claim to have brain damage or to have lost large chapters of the memories of their lives, they must be lying.”
In one of the most revealing points of CEO Nicol’s deposition, she was asked if she knew what the point is of sending electricity through a brain “if it’s not just to cause a convulsion?” Her answer was simple: “No.”
MECTA filed for bankruptcy in 2021. In its filing, the company cited lawsuits related to the company’s SpECTrum device. The lawsuits were filed by patients seeking damages over brain damage and serious memory loss allegedly caused by electroshock. MECTA claimed that, due to the litigation, the company was unable to obtain product liability insurance to cover the SpECTrum device, and thus was forced to stop manufacturing and servicing the product as of September 1, 2021.
MECTA told the bankruptcy court that its remaining “innovative ∑igma™ device is different from SpECTrum, and is insured and cleared by the FDA as a Class II medical device.” In truth, given the device necessarily is a “grandfathered” device (see below), there cannot possibly be a meaningful difference between the SpECTrum and ∑igma™ devices. MECTA’s maneuver of withdrawing one device from the market and replacing it with a new device, in an apparent attempt to avoid liability, is unlikely to succeed since the mechanism of action of the respective devices and the damage they cause are the same.
ECT History
In the late 1930s, Ugo Cerletti was the chair of the Department of Neuropsychiatry at the University of Rome. After observing slaughterhouses apply electricity to pigs to render them manageable for slaughter, he theorized that electricity could be used to treat psychosis. Cerletti and his colleague, Lucino Bini, began to test the theory by applying electricity to dogs. Bini noted that there was a high mortality rate in Cerletti’s dogs following the experiments, however proceeded to conduct experiments in humans beginning in 1938.
In April 1938, Cerletti and Bini applied ECT to the first human patient. A 40-year-old man who had been found wandering the train station in Rome and speaking gibberish was brought to the University of Rome and Cerletti and Bini applied 70 volts of electricity to his temple. It has been reported that, while the scientists were deliberating whether they should apply a second higher voltage, the patient pleaded “Non una seconda! Mortifera!” (“not again it will kill me!”).
Cerletti publicly presented his results on the use of ECT on this patient at the Medical Academy of Rome, claiming it could cure the insane. Starting in the early 1940s, ECT began to gain acceptance for the purported treatment of schizophrenia (and eventually other psychiatric ailments) across Europe and in the United States.
A modified version of ECT was introduced in the 1950s to prevent the damage caused by the ECT-induced convulsions, namely, patients received muscle relaxants and general anesthesia. This also gave researchers the opportunity to evaluate ECT against a simulated ECT control group (patients who solely received general anesthesia and no ECT). The first of these studies in 1953 found no difference in the efficacy between the ECT group and the control group. In 1959, scientists studied outcomes for ECT and depression in addition to schizophrenia. The placebo-controlled study again found no significant difference in effectiveness of alleviating symptoms between ECT and simulated ECT, for depression or schizophrenia.
Medical Studies Have Demonstrated ECT Causes Brain Damage
ECT and brain damage have been inextricably linked since inception. ECT’s early advocates believed brain damage was actually the reason the treatment was effective. In 1941, Dr. Walter Freeman, a proponent of lobotomies, wrote a paper titled “Brain Damaging Therapeutics,” in which he stated:
“The greater the damage, the more likely the remission of psychotic symptoms … Maybe it will be shown that a mentally ill patient can think more clearly and more constructively with less brain in actual operation.”
The idea that brain damage can be therapeutic in this day and age seems far-fetched. But some still accept that brain damage caused by ECT has medical benefits. In 2012, research scientists reported that ECT reduced “functional connectivity” in the brain. But rather than condemning the ECT, the study authors argued that this damage served as evidence that the brains of patients with depression have “hyperconnectivity” that ECT corrects.
Another study from 2013 found that ECT is effective at targeting and erasing harmful memories. The authors go so far as to suggest that ECT should be used for this purpose.
The textbook “Preventable Brain Damage” cites different types of studies that have shown brain damage resulting from ECT (including animal studies, human brain autopsy reports, subjective reports long after the administration of ECT and psychological testing in patients with a history of ECT).
According to one animal study cited in the book, significant differences were noted in cats who received ECT, which showed “clearly irreversible changes such as shadow cells and neuronophagia.”
Psychological testing of patients with a history of ECT treatments showed the “ECT patients were significantly inferior on all three tests,” and “the research using psychological tests with patients with history of many ECTS does suggest permanent impairment.” The study author concluded:
“There seems to be little doubt that ECT has, at least in the past, caused permanent brain damage in some patients and has the capacity to continue to do so.”
In 1979, a study in Archives of General Psychiatry documented that cerebral atrophy was significantly more common in patients who received ECT shock therapy.
In 1981, a brain scan study confirmed that brain shrinkage was significantly more common among patients who received ECT compared to other patients.
In 1986, a study found that ECT recipients were twice as likely to have a measurable loss of brain tissue in the front area of the brain and a tripling of the incidence of a loss of brain tissue in the back of the brain.
In 1990, scientists analyzing MRI scans of patients found a strong correlation between previous ECT treatments and loss of brain tissue.
Medical Studies Have Demonstrated ECT Causes Permanent Memory Loss, Amnesia
A 2003 review of studies of self-reported memory loss among those at least six months post ECT found a range  of patients reporting memory loss from 51% to 79%, with an average of 70%. The same review also found that the range for “persistent or permanent memory loss” was 29–55% with an average of 38%.
of patients reporting memory loss from 51% to 79%, with an average of 70%. The same review also found that the range for “persistent or permanent memory loss” was 29–55% with an average of 38%.
In 2004, a report from the New Zealand government concluded that “ECT may permanently affect memory,” and bemoaned the “slowness in acceptance by some professional groups that such outcomes are real and significant in people’s lives.” The psychiatry textbook Introductory Textbook of Psychiatry
In 2007, the first large-scale prospective study of cognitive outcomes of electroconvulsive therapy found that six months after ECT, autobiographical memory was significantly worse (p <.0001) than pre-ECT levels. Additionally, 12.4% suffered “marked and persistent retrograde amnesia.” This same study also found that women were 2.5 times more likely than men to be categorized as “marked and persistent retrograde amnesia.”
In 2019, the authors of The Cognitive Effects of Electroconvulsive Therapy: A Critical Review reported, “[r]ecent meta-analyses suggest the most prominent deficits are on measures of attentional/executive control (i.e., tests measuring cognitive flexibility, inhibitory control, and processing speed) and auditory verbal learning/recall (i.e., unstructured list learning), a memory task that is also strongly correlated with executive functioning.”
A report from the American Psychiatric Association notes, “evidence has shown that ECT can result in persistent or permanent memory loss.” A subsequent review of the research confirmed that “ECT can cause persistent or permanent memory loss, especially autobiographical memory.”
ECT Death Studies
Numerous studies have found that the mortality rates for ECT patients is many times greater than the rate promulgated by the American Psychiatric Association, which indicates that the ECT death rate is “1 per 10,000 patients, or 1 per 80,000 treatments.”
In one study of ECT patients in Texas, authors reported that of the 8,148 ECT recipients, seven died within 48 hours of treatment. Excluding two deaths considered “unlikely to have been related to ECT,” this amounts to one per 1,630. Eight more patients died within two weeks of a “cardiac event,” a common ECT-related cause of death. If these are included, the mortality rate becomes one per 627.
A 2019 review of more than 80 studies found that about one in 50 people suffer “major adverse cardiac events” after ECT.
Electroconvulsive Therapy is Still Used Today Without Proof of Safety and/or Efficacy
From the early days of electroconvulsive therapy to the present day, psychiatric experts have documented brain damage and memory loss correlated with ECT. A vocal “ECT survivor community” has been voicing their objection to the continued use of shock treatment for decades.
Between 2009 and 2011, the FDA opened a public docket seeking reports of adverse events related to ECT. Thousands of adverse event complaints were submitted, hundreds of which alleged serious brain injury. Following a 2011 FDA hearing, the FDA provided “An Executive Summary” containing information on the available safety and effectiveness data for ECT in treating various forms of severe psychiatric illness. According to the FDA, memory loss, including autobiographical memory loss (memory loss of personal events and self-identity), was one of the “most concerning” adverse events associated with ECT.
As for the efficacy of ECT, researchers conducted an analysis of all ECT studies meeting the criteria for the highest and most conclusive level of evidence in medicine: randomized, prospective, double-blind placebo-controlled trials of ECT (conducted by others, not by ECT manufacturers). These studies compared real ECT with “sham” ECT. According to the researchers, the studies “provide definitive evidence that real ECT is no more effective than sham ECT.”
In a meta-analysis of pre-existing ECT studies conducted by Irving Kirsch of Harvard University and John Read and Laura McGrath of the University of East London, the authors concluded:
“Given the high risk of permanent memory loss and the small mortality risk, this longstanding failure to determine whether or not ECT works means that its use should be immediately suspended until a series of well designed, randomized, placebo-controlled studies have investigated whether there really are any significant benefits against which the proven significant risk can be weighed.”
ECT Dosing
Dose optimization of ECT remains controversially “unreconciled despite decades of research.”
“After more than 80 years of use, ECT proponents haven’t come up with standardized protocols based on safety studies. Nor can they. Between more than 12 dosing variables involved plus the individual patient’s anatomic differences, it literally creates infinite dosing variables with potentially catastrophic risk. Due to the number of variables involved in its administration, ECT is basically the equivalent of medically sanctioned Russian Roulette.” – Sarah Price Hancock, Life After ECT
“Due to the number of variables involved in its administration, ECT is basically the equivalent of medically sanctioned Russian Roulette.”
Is ECT Approved by the FDA?
No. ECT devices were “grandfathered” in before a new law would have required their approval. They remain on the market unapproved because of a troubling and dangerous loophole.
Before Congress enacted the comprehensive Medical Device Amendments of 1976 to the Federal Food, Drug, and Cosmetic Act, medical devices could be marketed and sold without prior approval from the U.S. FDA. The Federal Food, Drug, and Cosmetic Act, as amended by the Medical Device Amendments of 1976, established a framework for regulating medical devices by placing them into one of three risk-based categories before they are allowed to enter the U.S. market:
- Class I Medical Devices: Present no unreasonable risk of illness or injury and are subject to regulation through “general controls.” This includes such devices as tongue depressors and bandages.
- Class II Medical Devices: Present potentially more harmful risks and are subject to general controls. FDA has authority to require Class II devices comply with other “special controls” or performance standards.
- Class III Medical Devices: Present “a potential unreasonable risk of illness or injury.”
New medical devices, including those not in commercial distribution before 1976, are classified automatically as Class III without any FDA rulemaking process. Before a Class III device may be introduced into the market, a manufacturer must obtain “premarket approval,” which requires the device manufacturer to submit information to FDA that provides reasonable assurance that the device is safe and effective for its intended use.
Premarket approval is the most detailed type of device marketing application and review the FDA requires. A premarket approval application must include sufficient valid scientific evidence to assure that the device is safe and effective for its intended use(s). It also requires clinical testing.
However, a loophole known as the “grandfathering” provision permits Class III devices that were on the market before the 1976 Medical Device Amendment’s enactment to remain on the market until FDA initiates and completes rulemaking requiring device manufacturers to submit a premarket approval application.
In addition, Congress created another loophole which permits new manufacturers to distribute similar devices by showing through a premarket notification process that their new devices are “substantially equivalent” to grandfathered devices. A device is “substantially equivalent” to a grandfathered device only if, among other things, the device has the same “intended use” as the predicate device.
According to the ECT lawsuits, it is this grandfathering loophole that has allowed ECT manufacturers like Somatics and MECTA to keep their dangerous machines on the market. Because ECT machines were on the market prior to the 1976 enactment of the Medical Device Amendments, ECT companies obtained grandfathering clearance for ECT devices without submitting a premarket approval application and without having to submit any clinical trials concerning the safety and efficacy of ECT devices.
For decades, the FDA’s position on ECT machines has been clear: “The long-term safety and effectiveness of ECT treatment has not been demonstrated."
In issuing its clearance to Somatics, the FDA on multiple occasions, informed Somatics that:
This letter does not in any way denote official FDA approval of your device or its labeling. Any representation that creates an impression of official approval of this device because of compliance with the premarket notification is misleading and constitutes misbranding. (emphasis added)
Resources (ECT Court Documents – Legal Briefs, Internal Documents, and Depositions)
ECT Expert Reports
Expert Report of Dr. Janet Arrowsmith
- Former Deputy Director U.S. Food and Drug Administration’s AIDS Coordination Staff
- Board Certified in Internal Medicine
- Elected Fellow of the American College of Physicians
- Elected Fellow of the American College of Epidemiology
According to Dr. Arrowsmith’s report, despite roughly 80 years of use worldwide, “there are no blinded, appropriately powered, adequate, and well-controlled studies published in the medical literature or submitted by ECT device manufacturers supporting the efficacy or safety of electroconvulsive therapy in psychiatric patients.”
Expert Report of Dr. Kenneth Castleman
- Biomedical Electrical Engineer
- Senior Scientist U.S. National Aeronautics and Space Administration’s Jet Propulsion Laboratory (retired)
- Served on the Faculty at Caltech
- Adjunct Professor at the University of Texas
- Research Fellow at the University of Southern California and the University of California, Los Angeles
According to Dr. Castleman’s report, “ECT has the potential to injure or kill brain cells by at least two different electrical mechanisms, heating and electroporation. The scientific literature has demonstrated brain damage in earlier times, and recent studies using high magnet-strength MRI show ECT-induced changes in the sizes of certain brain structures. Little is known about whether damage on a cellular level is continuing to occur with modern ECT devices and practice. Further, studies to evaluate the risk of electroporation by ECT have not been reported. Despite its widespread use, ECT exposes patients to risks of brain damage that have not been thoroughly evaluated. The opinion of ‘authorities in the field’ is being substituted for scientific fact.”
Expert Report of Dr. Bennet Omalu
- Clinical Pathologist, Anatomic Pathologist, Forensic Pathologist, Neuropathologist, and Epidemiologist
- President and Medical Director of Bennet Omalu Pathology and a Clinical Professor at the Department of Medical Pathology and Laboratory Medicine at the University of California, Davis.
- Received the “Distinguished Service Award” from the American Medical Association, the AMA’s most prestigious award.
- Life and work featured in the Hollywood film “Concussion,” where actor Will Smith portrayed Dr. Omalu’s work as the first person to discover chronic traumatic encephalopathy (CTE) in football players.
According to Dr. Omalu’s report, “[t]he amounts of electrical energy introduced to the human brain by ECT machines can be nothing but harmful and dangerous. These mounts of energy are exponentially outside the tolerable homeostatic ranges and thresholds of the human brain and nervous system and will no doubt, and are in fact expected to cause cellular physiologic, biochemical, and anatomic injuries to the human brain. Since the human brain is post-mitotic and cannot completely regenerate itself following such injuries, and since such ECT-induced electrical injuries occur repeatedly over time, the patient who receives ECT therapy will manifest permanent and cumulative brain injury, which can be progressive over time and result in chronic encephalopathies and brain degeneration. Such brain degeneration can manifest with multi-domain neurological impairments including cognitive impairment, mood disorders and neuro-psychiatric impairment, behavioral impairment, motor disorders and other somatic symptoms and signs.”
Expert Report of Dr. John Read
- Professor of Clinical Psychology at the University of East London, England
- Clinical Psychologist and manager of mental health services, in the United States, England, and New Zealand, predominantly with patients at the extreme end of the spectrum of psychiatric disturbance.
- Has over 200 research publications (Google Scholar 4.12.2021), which have been cited over 17,000 times, published over 50 book chapters, edited or authored five books, and given over 60 invited plenary/keynote addresses at psychiatric conferences in 17 countries.
- Published four reviews of the research literature on the efficacy, safety, and mechanisms of action of electroconvulsive therapy.
According to Dr. Read’s report, there is “little evidence that ECT is more effective than placebo in the short-term, and none at all in the long-term. ECT does not prevent suicide. ECT causes persistent/permanent memory loss and brain damage in a substantial proportion of recipients, somewhere in the range of 12% to 55%. This damage takes the form of both anterograde and retrograde amnesia. The number of electroshocks received is predictive of the degree of damage done by ECT.
Our Case Results

-
$265 Million Settlement Fatal Train Crash
In 2016, Wisner Baum attorney Timothy A. Loranger and six other attorneys in the Plaintiffs’ Management Committee were able to secure a $265 million settlement for victims of the 2015 Amtrak 188 derailment in Philadelphia, one of the largest in the U.S. for 2016.
-
$14 Million Settlement A Major US Plane Crash
Wisner Baum obtained a $14 million settlement for the death of a passenger in a major US plane crash.
-
$12 Million Settlement Helicopter Crash
Wisner Baum secured a $12 million settlement for a passenger who was injured in a helicopter crash.
-
$10 Million Settlement A Major Foreign Plane Crash
Wisner Baum obtained a $10 million settlement for the death of a passenger in a major foreign plane crash.
-
$2.0 Billion Verdict Personal Injury
In May of 2019, the jury in the case of Pilliod et al. v, Monsanto Company ordered the agrochemical giant to pay $2.055 billion in damages to the plaintiffs, Alva and Alberta Pilliod, a Bay Area couple in their 70s.
-
$80 Million Verdict Personal Injury
Wisner Baum attorneys served on the trial team in the case of Hardeman v. Monsanto Company, which resulted in an $80 million jury verdict for the plaintiff, Edwin Hardeman.

Client-Focused Representation
REVIEWS & TESTIMONIALS
We believe our track record speaks for itself. But you don’t have to take our word for it. See what our clients have to say about working with us.
-
"I Can’t Imagine a Better Law Firm"
Multiple lawyers recommended Wisner Baum to me and I have been consistently impressed with the quality of their work.
- Best Law Firms Survey -
"They Are About Changing the Systems..."
Wisner Baum are not only amazing attorneys but more importantly, they are activists. They are about changing the systems which got us into trouble in the first place. They understand their role in the process of making change.
- Kim Witczak -
"Top Legal Minds in the Country"
The Wisner Baum firm has some of the top legal minds in the country; they are driven, determined, trustworthy, ethical and passionate.
- From Best Lawyers® Best Law Firms





