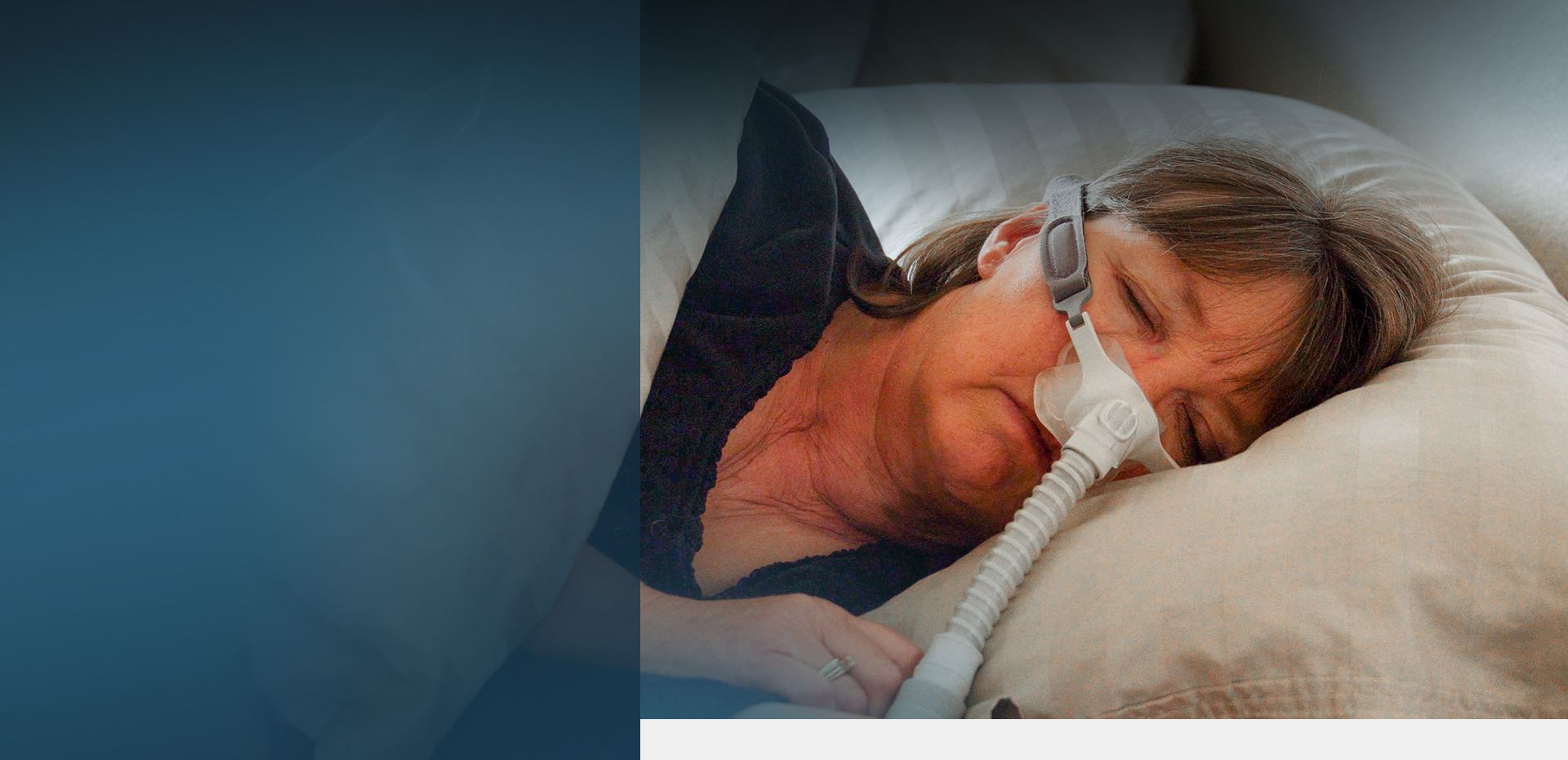
Philips CPAP Lawsuit
Philips Respironics announced a recall for sleep apnea machine models that may increase the risk of developing lung cancer, kidney cancer, liver cancer, and other forms of cancer. The affected products include continuous positive airway pressure (CPAP) machines, Bilevel positive airway pressure (BiPAP) machines, and mechanical ventilators designed to help people with breathing disorders, including sleep apnea. The U.S. Food and Drug Administration (FDA) identified the Philips Respironics recall as the agency’s most serious (Class I).
The Philips recall affects millions of people who rely on these machines to help them with breathing disorders. According to Philips, roughly 80% of the affected products are CPAP machines for people with sleep apnea. Shocked by the news that these products may be linked to cancer and a host of other serious health issues, many are turning to the courts to seek damages against Philips for putting a defective and dangerous product on the market.
August 23, 2024: We have important announcements regarding the Philips Respironics Settlement agreement.
To participate in the CPAP personal injury settlement program, claimants must have been included on an Identification Order Declaration of Primary Counsel and Eligible Claimant List with a Qualifying Injury on or before June 21, 2024. Claimants must also have been retained as a client by an attorney on or before April 29, 2024.
Unrepresented eligible claimants may participate if they have filed a CPAP lawsuit alleging a qualifying injury in the MDL Court or Massachusetts Court or submitted an Identification Order Declaration with a qualifying injury on or before June 21, 2024. You can read about qualifying injuries and who is considered an eligible claimant in the master settlement agreement.
To receive compensation from the CPAP settlement, all eligible claimants must submit a Registration Packet on or before December 10, 2024.
June 11, 2024: Over 790 CPAP lawsuits are pending in the multidistrict litigation alleging injuries related to recalled Philips breathing machines. If the proposed settlement from earlier this year is not approved, bellwether trials in the MDL could take place in 2025 before U.S. District Judge Joy Flowers Conti.
May 1, 2024: Philips announced a personal injury settlement worth nearly $1.1 billion to resolve lawsuits involving its CPAP machines, which were the subject of a large-scale recall in 2021. The settlement will compensate individuals in the U.S. who filed lawsuits against the company, alleging the defective breathing machines caused harm. The company announced it would also pay an additional $25 million for medical monitoring as part of the settlement.
Philips did not admit to fault or liability for any injuries alleged in the medical device lawsuits. Following the announcement, Philips experienced a surge in its stock value in response to the settlement news and its first-quarter financial results.
The settlement excludes a previous $600 million settlement to compensate affected device owners. Philips is offering $100 for each recalled device for consumers still possessing the affected machines. Affected CPAP owners have until August 9, 2024 to pursue a claim.
The announced settlement is not yet final. Payments are expected to be disbursed to those who filed lawsuits in 2025.
September 13, 2023: Last week, Philips Respironics agreed to a partial CPAP settlement worth approximately $479 million to settle class action claims regarding flaws in the company's CPAP breathing machines. The CPAP settlement specifically addresses monetary reimbursements for device users and vendors who financed replacements, with no cap on economic claims that can be made. This means that other CPAP users may be able to qualify for compensation.
The agreement does not cover other claims related to personal injuries or medical expenses resulting from the use of the machines. Those claims will be litigated separately. Some legal experts believe the personal injury and wrongful death cases could be worth billions.
Under the terms of the CPA class action settlement announced last week, affected consumers will receive compensation ranging from approximately $50 to $1,500. Additionally, Philips will provide $100 for each device returned. The company has revealed that as of September 2023, it has already replaced and delivered nearly 2.5 million devices to U.S. consumers and suppliers.
Philips has been the subject of public scrutiny for failing to promptly inform consumers about the potential flaws in their CPAP breathing machines. Records show that concerns were raised within the company as early as 2015. The FDA has received over 105,000 reports of injuries and 385 reports of deaths possibly linked to the foam degradation in Philips CPAP machines.
What Caused the Philips CPAP Recall?
Philips Respironics cited a serious problem with the polyester-based polyurethane foam that dampens the sound and vibration when the machines are in use. The foam can break down and release small chemical particles into the machine’s airway, which can then be inhaled or ingested. According to the FDA, particulate exposure from Philips sleep apnea machines may have “toxic or carcinogenic effects to organs, such as kidneys and liver.”
CPAP Class Action Lawyers
If you or someone in your family developed cancer after using any of the Philips Respironics CPAP machines, BiPAP machines, or ventilators listed below, you may be eligible to pursue justice and compensation in a lawsuit against the manufacturer. CPAP lawyers represent victims on a contingency fee basis, which means the attorneys only get paid if your case ends in a successful verdict or settlement. If for some reason the litigation does not result in compensation, you would not owe anything.
To learn more about your legal rights and see if you qualify for a CPAP lawsuit, fill out our case evaluation form below or call us at (855) 948-5098.
Which CPAP Machines Did Philips Recall?
According to the U.S. Food and Drug Administration (FDA), the following Philips CPAP machines, BiPAP machines, and ventilators have been recalled:
Recalled CPAP and BiPAP Products:
- C-Series ASV
- C-Series S/T and AVAPS
- Dorma 400
- Dorma 500
- DreamStation
- DreamStation ASV
- DreamStation Go
- DreamStation ST, AVAPS
- E30 (Emergency Use Authorization)
- OmniLab Advanced+
- REMstar SE Auto
- SystemOne (Q-Series)
- SystemOne ASV4
Recalled Ventilators:
- A-Series BiPAP A30
- A-Series BiPAP A40
- A-Series BiPAP Hybrid A30*
- A-Series BiPAP V30 Auto
- Garbin Plus, Aeris, LifeVent
- Trilogy 100
- Trilogy 200
*not marketed in the U.S.
Why Are CPAP Sleep Apnea Machines Recalled?
Philips determined a recall was appropriate after receiving user reports and conducting lab testing on several CPAP, BiPAP, and ventilators, including the widely-used Philips DreamStation. The company found that the polyester-based polyurethane (PE-PUR) sound abatement foam used in Philips sleep machines can degrade and deteriorate over time, which can cause people who rely on these machines for breathing assistance to inhale or ingest small particles or gas.
Inhaling or ingesting PE-PUR particulate can cause a host of health issues. Philips has already acknowledged that the risks of particulate exposure include:
- Adverse effects to kidneys and liver
- Asthma
- Headache
- Inflammatory response
- Irritation (eye, respiratory tract, and skin)
- Respiratory issues
- Toxic carcinogenic effects
According to Philips, the foam degradation may worsen in certain conditions, including higher temperatures and humidity, and the use of unapproved cleaning methods (including ozone).
If you are interested in learning more about pursuing a lawsuit against Philips, contact us today or call (855) 948-5098. Our class action attorneys offer free and confidential CPAP lawsuit case evaluations.
CPAP Lawsuit FAQ
-
What is a CPAP Machine?
CPAP (continuous positive airway pressure) machines are medical devices used to treat people with obstructive sleep apnea. CPAP sleep machines send a steady flow of oxygen to the nose and mouth, keeping the airway open and allowing the patient to breathe normally. CPAP machines have motors that generate oxygen, which travels through a filter and a flexible tube before reaching the mask, sealed around the patient’s nose and mouth.
-
What is the Difference Between CPAP and BiPAP?
They may look similar, but BiPAP (bilateral positive airway pressure) machines differ from CPAP machines. BiPAP machines are more complex than CPAP machines in that they have two pressures (inhalation and exhalation) compared to the CPAP’s one pressure. BiPAP therapy is the preferred treatment method for people who do not respond to CPAP therapy, including those with central sleep apnea, complex sleep apnea, or chronic obstructive pulmonary disease (COPD). BiPAP machines are generally more expensive than CPAP machines, so many people who can be treated by either choose CPAP instead of BiPAP. -
What is Sleep Apnea?
Sleep apnea is a serious sleeping disorder where a person’s breathing repeatedly starts and stops. It is the most common sleeping disorder in the U.S.
There are three types of sleep apnea:
- Obstructive Sleep Apnea – Obstructive sleep apnea happens when an individual’s throat muscles intermittently relax, blocking the airway during sleep. It is the most common type of sleep apnea, affecting an estimated one in 15 people in the U.S.
- Central Sleep Apnea – Central sleep apnea happens when an individual’s brain does not send the proper messages to the muscles that control breathing. This study estimates that .9% of people over 40 have central sleep apnea.
- Complex Sleep Apnea Syndrome – Complex sleep apnea, also known as treatment-emergent central sleep apnea, occurs when a person presents with both obstructive sleep apnea and central sleep apnea.
-
How Do Philips Breathing Machines Cause Cancer?
The polyester-based polyurethane foam used in the recalled Philips breathing machines may degrade over time. This degradation may allow particles to flow into the affected CPAP, BiPAP, and ventilator air pathways, which then causes the person using the device to breathe in the particles. As Philips said in its clinical information, the “absence of visible particles does not mean that foam breakdown has not already begun.” This means that even if you can’t see foam degradation on your sleeping machine, you still may be inhaling toxic particles. Philips has already admitted the presence of the following:
- Toluene Diamine: According to the U.S. Environmental Protection Agency (EPA), in animal studies, toluene-2,4-diamine was found to be a carcinogen following dietary administration. EPA noted a significant increase in the incidence of a many different tumor types, including liver, mammary gland, subcutaneous fibromas, lung lymphomas, and leukemia.
- Toluene Diisocyanate (TDI): The International Agency for Research on Cancer (IARC), the gold standard for carcinogenicity testing, classifies TDI as a possible human carcinogen. IARC came to its conclusion based on sufficient evidence in experimental animals and inadequate evidence in humans. This study notes that TDI is a “well-known sensitizer and cause of occupational asthma.”
- Diethylene Glycol: Associated with renal failure (acute kidney injury).
- Dimethyl Diazene: Also known as azomethane. The oxide derivative of this compound is azoxymethane, a known carcinogen.
- Phenol, 2,6-bis (1,1-dimethylethyl)-4-(1-methylpropyl): According to EPA, Phenol is considered toxic to humans via oral exposure. Anorexia, a dark coloration of the urine, diarrhea, progressive weight loss, salivation, vertigo, and blood and liver effects have been reported in chronically (long-term) exposed humans. Animal studies have shown reduced fetal body weights, growth retardation, and abnormal development in the offspring among animals exposed to phenol by the oral route.
-
Can I File a CPAP Lawsuit Against Philips?
If you were diagnosed with cancer after using one of the affected Philips recall products above, you may be eligible to pursue compensation in a CPAP lawsuit. The fastest way to find out more about your eligibility is to contact our firm as soon as you are able. Our legal team will evaluate your case and let you know if you can proceed with a claim.
Our Case Results

-
$265 Million Settlement Fatal Train Crash
In 2016, Wisner Baum attorney Timothy A. Loranger and six other attorneys in the Plaintiffs’ Management Committee were able to secure a $265 million settlement for victims of the 2015 Amtrak 188 derailment in Philadelphia, one of the largest in the U.S. for 2016.
-
$14 Million Settlement A Major US Plane Crash
Wisner Baum obtained a $14 million settlement for the death of a passenger in a major US plane crash.
-
$12 Million Settlement Helicopter Crash
Wisner Baum secured a $12 million settlement for a passenger who was injured in a helicopter crash.
-
$10 Million Settlement A Major Foreign Plane Crash
Wisner Baum obtained a $10 million settlement for the death of a passenger in a major foreign plane crash.
-
$2.0 Billion Verdict Personal Injury
In May of 2019, the jury in the case of Pilliod et al. v, Monsanto Company ordered the agrochemical giant to pay $2.055 billion in damages to the plaintiffs, Alva and Alberta Pilliod, a Bay Area couple in their 70s.
-
$80 Million Verdict Personal Injury
Wisner Baum attorneys served on the trial team in the case of Hardeman v. Monsanto Company, which resulted in an $80 million jury verdict for the plaintiff, Edwin Hardeman.

Client-Focused Representation
REVIEWS & TESTIMONIALS
We believe our track record speaks for itself. But you don’t have to take our word for it. See what our clients have to say about working with us.
-
"I Can’t Imagine a Better Law Firm"
Multiple lawyers recommended Wisner Baum to me and I have been consistently impressed with the quality of their work.
- Best Law Firms Survey -
"They Are About Changing the Systems..."
Wisner Baum are not only amazing attorneys but more importantly, they are activists. They are about changing the systems which got us into trouble in the first place. They understand their role in the process of making change.
- Kim Witczak -
"Top Legal Minds in the Country"
The Wisner Baum firm has some of the top legal minds in the country; they are driven, determined, trustworthy, ethical and passionate.
- From Best Lawyers® Best Law Firms





